Notably, intestinal cancer is inextricably linked to gut inflammation, and Crohn’s Disease, an inflammatory bowel disease, is associated with colorectal cancer development. Several mechanisms are involved in this relationship, from innate and adaptive immune cell migration to endocrine and neural pathways, translocation of bacteria or bacterial products and toxins, and systemic inflammation and oxidative stress modulation. Gut microbiota seems to play an important role in the development of these and other inflammationassociated diseases and conditions. The outgrowth of opportunistic bacteria can drive an increase in inflammation, and the loss of benign bacteria reduces immunoregulation.
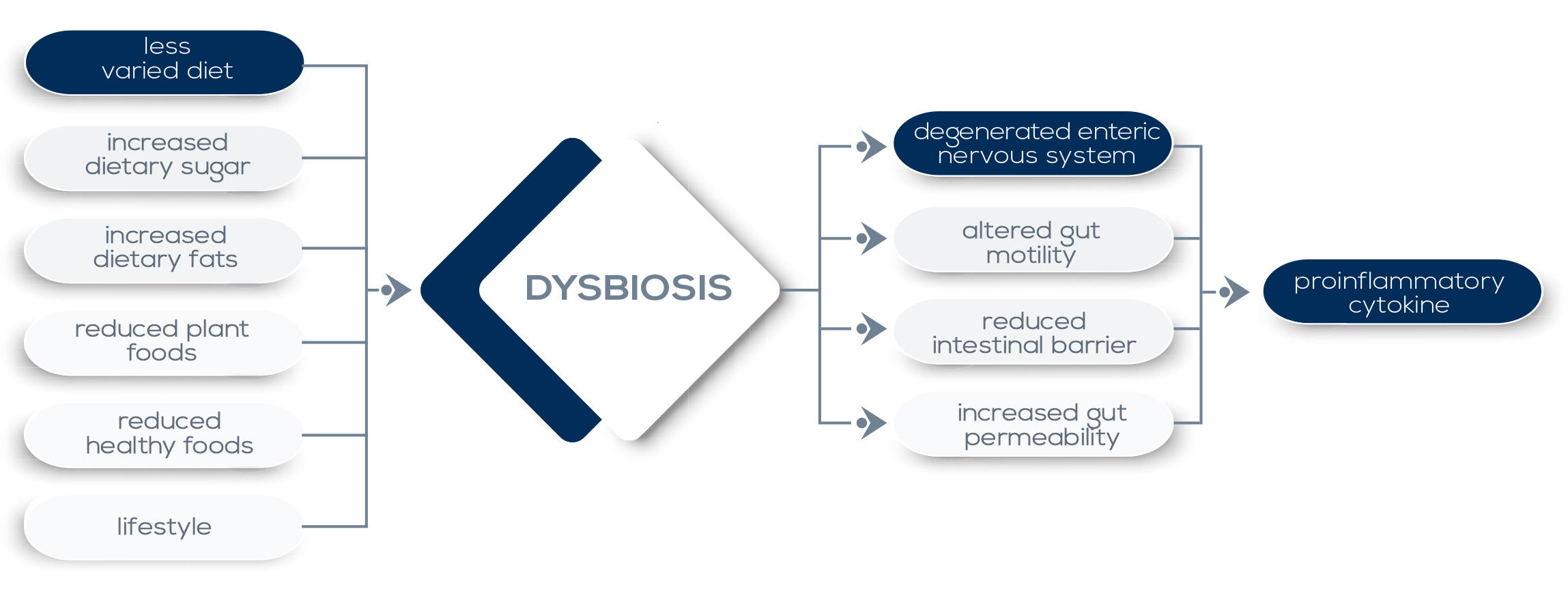
Dominance of bacteria characterized by enhanced invasiveness and inflammatory properties (in particular γ-proteobacteria) can directly exacerbate inflammation and tissue damage, and mechanisms such as their capability to thrive on metabolites derived from inflammatory settings could contribute to their proliferation. Moreover, external factors such as the overuse of antibiotics, changes in diet, and the elimination of chronic parasitic infections may select for microbiota lacking the resilience required for the establishment of a balanced microbiotahost interaction in which stimulatory and regulatory signals allow for immunity development without compromising the tolerance to innocuous antigens.
Such a selection is now believed to contribute to the increase in chronic inflammatory and autoimmune disorders seen in westernized countries. In fact, many inflammatory diseases are associated with significant alterations in the resident microbiota, shifting from a healthy to a diseased state. Susceptibility to some diseases could be at least in part due to the presence of specific bacteria particularly adept at surviving in, and contributing to, inflammation. Evidence of this phenomenon comes both from mice and human studies. In particular, the development of Inflammatory Bowel Diseases (IBDs, namely Crohn’s disease – CD – and ulcerative colitis – UC) is believed to be the result of a combination of genetic factors, the immune system’s activity, and environmental factors – including the microbiota. Both CD and UC are associated with a reduced complexity of the microbiota and with a dysbiosis characterized by the outgrowth of proteobacteria (in particular, the Enterobacteriaceae family).
Moreover, in CD patients, commensal microbiota that are intrinsically inflammatory (such as Escherichia coli, Yersinia and Clostridium difficile) are much more common than in healthy individuals, and there is an increase in serum antibodies against the microbiota. Currently, it is thought that IBDs are caused or exacerbated by a loop where host mutations leading to dysregulated immune responses in the gut drive the outgrowth of bacteria that promote more inflammation. In parallel, the loss of bacteria producing SCFAs (that have been shown to limit gastrointestinal inflammatory processes) may contribute to gut inflammation. In particular, Clostridia – a class of Firmicutes that directly induces immune cells opposing colitis induction and ferments dietary fiber to SCFAs – are reduced in IBDs patients.