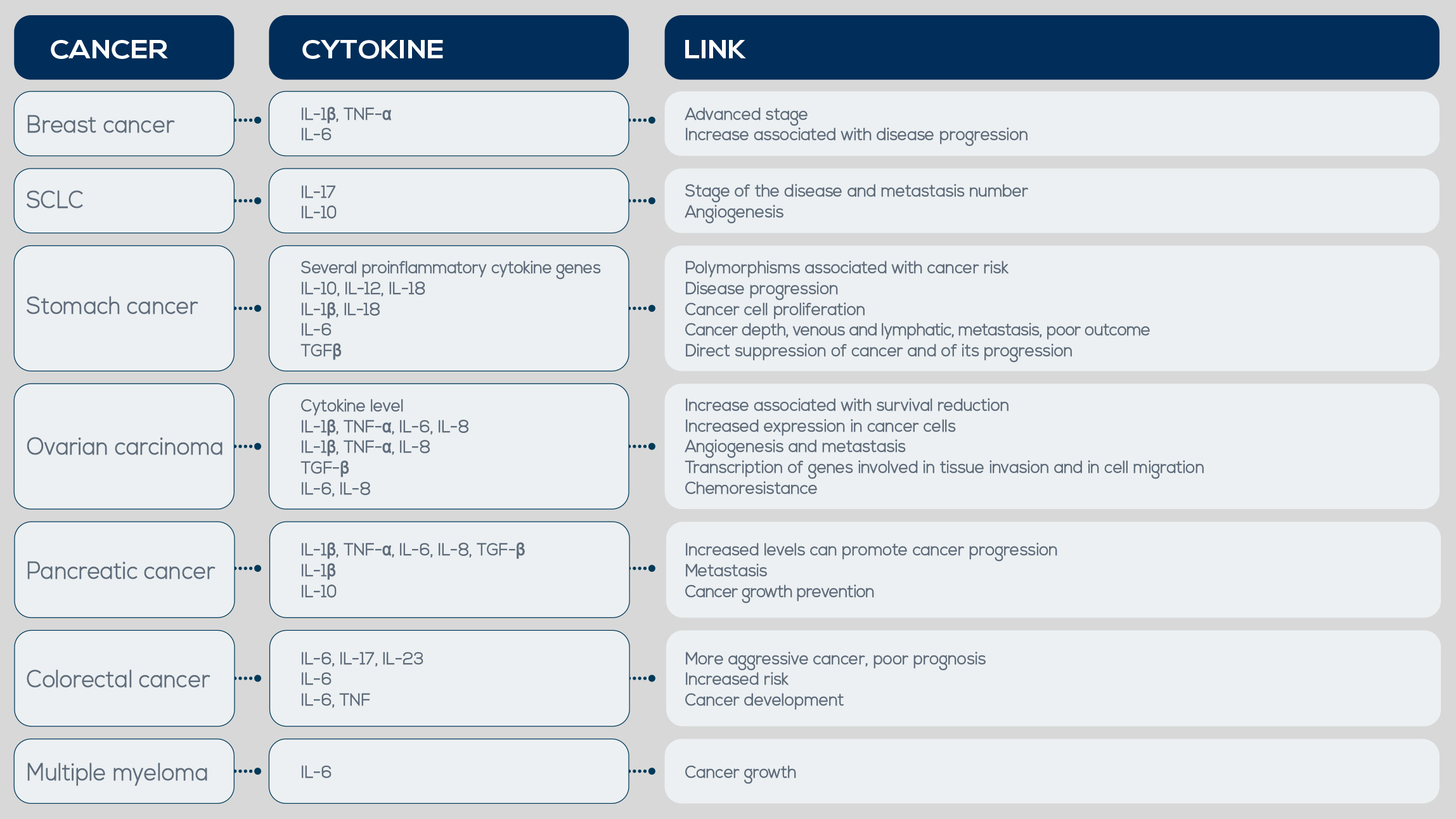
The ultimate goal of every type of inflammation is to return tissues to their normal state, and acute and chronic inflammation share many of the same effector molecules and cells involved in this task. Among other factors, pro-inflammatory cytokines are produced by activated inflammatory cells. If limited and shortlived, their expression is beneficial; however, the long-term expression of cytokines during chronic inflammation is detrimental and leads to chronic diseases, including cancer. Another important tumor-promoting factor is Tumor Necrosis Factor (TNF). It is produced during the initiation of inflammatory responses, plays a critical role in chronic inflammation maintenance, and is involved in tumor growth, angiogenesis, tissue remodeling, and metastasis. In breast carcinoma, for example, TNF-α serum concentration has been associated with a more advanced tumor phenotype.
Elevated expression of IL-17 and of another cytokine playing an important role in its expression, IL-23, has been detected in colon, ovaries, lung, breast, stomach, skin, liver, and head and neck cancer. Moreover, in small cell lung cancer IL-17 expression has been associated with tumor stage and metastases number, and it could be a new prognostic biomarker. These inflammatory cytokines along with others, can be upregulated and lead to exacerbation of tumor progression. They are increased in chronic pancreatitis (a condition associated with pancreatic cancer) and in early stages of cancer. IL-8 levels in chronic pancreatitis are 9.3 times more elevated than in normal pancreatic tissue, and in pancreatic cancer some polymorphisms in the IL-6 gene make its levels increase. IL-1β is involved in tumor growth and metastasis, and TNF-α can be used as a target for pancreatic cancer treatment.