Gut microbiota plays a fundamental role in the induction, education, and function of the immune system, establishing an alliance with its host’s defense weapons interweaving innate and adaptive immunity to select, calibrate, and terminate responses. The training of this system during infancy and childhood leads to the establishment of a durable relationship. The immune system continuously works to control this relationship, and the microbiota constantly reinforces the so-called “barrier immunity”, that is the mechanisms that allow its containment. These mechanisms consist of strategies to minimize the contact between microorganisms and bowel epithelial cells. First, intestinal cells produce a mucus layer that limits contact between the microbiota and host tissues and prevents microbial translocation in the bloodstream. Moreover, intestinal cells produce antimicrobial molecules too. Third, immune cells in the intestines produce antibodies (IgA) that are specific for microbiota antigens, and white blood cells in the intestinal wall (namely, macrophages) rapidly eliminate microbes translocating across the epithelial cell barrier. The body’s recognition of commensal microbiota is a critical step to be taken early on in life, during the preweaning period.
A certain degree of microbiota recognition is a common occurrence, in most cases it is not associated with a pathogenic response. However, gut dysbiosis – that is, alterations of gut microbiota – is associated with a number of health problems. Overall, allergic sensitization, eczema, and asthma are associated with a lower relative abundance of Bifidobacteriaceae and Lactobacillaceae and a greater relative abundance of Bacteroidaceae, Clostridiaceae, and Enterobacteriaceae. Meanwhile, a greater microbiota diversity promotes the development of a healthy immune system, reducing the risk of asthma and allergic diseases. However, gut microbiota’s role in human health goes far beyond immune system training: it is fundamental also for the immune system’s functioning under a steady state and inflammatory conditions, and for tuning the inflammation generated by cells involved in the control of pathogens in the bowel, protecting host tissues from damage. The mechanisms by which the microbiota shapes immunity are not yet completely understood. Commensal bacteria-derived signals could influence the expression of genes involved in innate responses, enabling a basal level of host-defense factors and a rapid response upon pathogens. What is known is that the microbiota directly and dynamically interacts with pathogens.
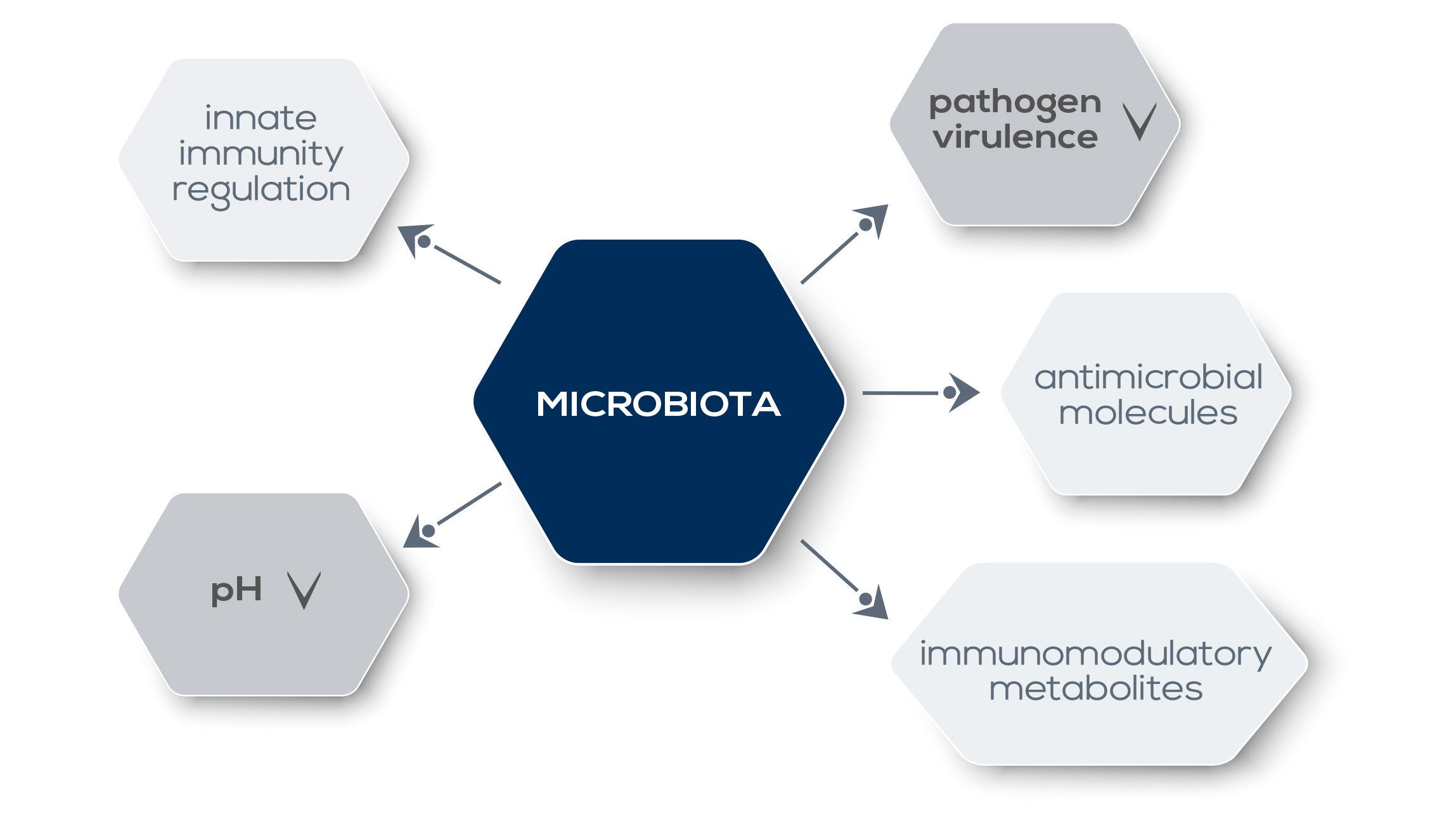
They compete for the same ecological niche; moreover, microbiota metabolites that modulate the activity of the immune system (such as short-chain fatty acids, SCFAs) can also downregulate the expression of pathogen virulence genes. Moreover, the microbiota promotes the establishment of a hostile environment for pathogens, for example by lowering local pH or by producing antimicrobial molecules. Alterations in gut microbiota can affect both local immune responses as well as immunity and inflammation in other organs, even if they are distant from the intestine. For example, a reduction of “good” gut bacteria via antibiotic treatment reduces the immune system’s response against intranasal influenza infection. This control on systemic immunity has profound consequences also in the context of therapy. Gut microbiota shapes and modulates the host immune system and plays a pivotal role in digestion and absorption of dietary components. For example, it can extract nutrients and energy from food fibers that would otherwise be lost because humans lack enzymes required to harvest them. Metabolites produced during such reactions can influence immune system functioning. Finally, gut microbiota participates in the detoxification of potentially dangerous substances, including carcinogens.