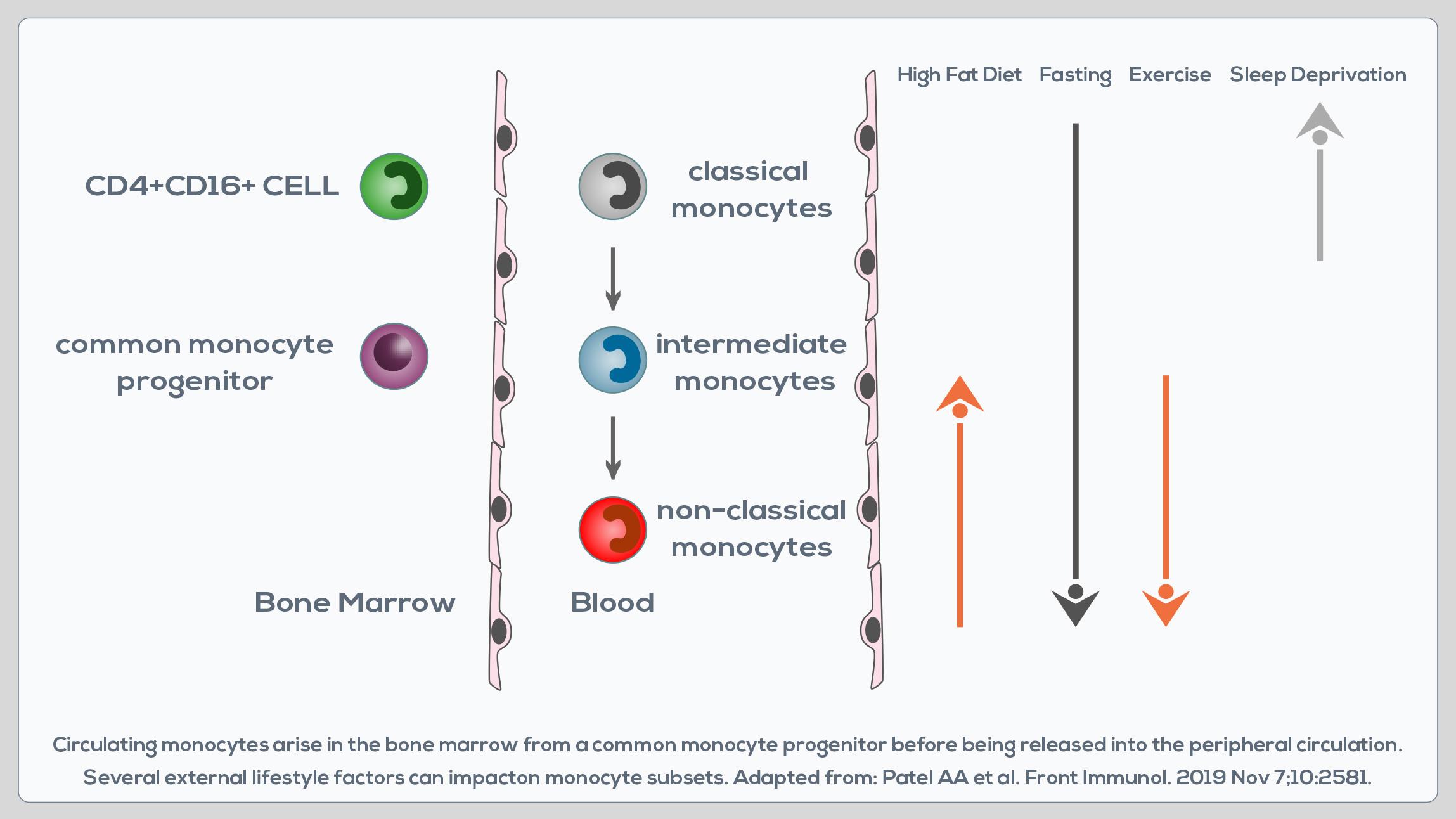
Monocytes are white blood cells developing in the bone marrow that are involved in both the innate immune response, and processes such as tissue repair. They circulate in the blood, migrating to sites of injury or infection after the engagement of chemokine and pathogen recognition receptors. They can perform pro-inflammatory and pro-resolving functions, take up cells and toxic molecules, and differentiate into inflammatory dendritic cells or macrophages. Two major monocyte subsets in the peripheral blood can be distinguished based on the differential expression of two molecules: CD14 and CD16. CD14++CD16- cells are known as “classical monocytes”, whereas CD14+CD16+ cells are known as “non-classical monocytes”. A third subset of monocytes is represented by CD14++CD16+ cells, or “intermediate monocytes”. CD14++CD16- cells represent up to 95% of monocytes in healthy individuals.
They are characterized by a high phagocytic capacity, innate sensing/ immune responses, and migration, and can be somewhat more efficient than CD14++CD16+ monocytes in producing reactive oxygen species (ROS) and constraining fungi. They can differentiate into monocyte-derived macrophages and dendritic cells, which are immune cells playing a pivotal role in the development and resolution of tissue inflammation. Compared to them, CD14++CD16+ cells display similar phagocytosis potential, but lower adhesion capacity, and greater class II molecule expression and IL-2 production. They are wellsuited for antigen presentation, cytokine production (TNF-α, IL-1β and IL-6), apoptosis regulation and differentiation. Their blood count is expanded in cases of systemic infection, so they must play an important role in the rapid response to pathogens. CD14+CD16+ monocytes are instead associated with wound healing and antiviral responses and promote neutrophil adhesion at the endothelial interface via TNF-α production. However, they do not produce the same levels of pro-inflammatory cytokines as classical monocytes.
Moreover, compared to classical CD14++CD16- cells, non-classical CD14+CD16+ monocytes express lower levels of chemokine receptors involved in monocyte migration. CD16+ subsets correlate with chronic inflammation-associated pathologies. Their absolute number and the relative contribution to the circulating monocyte pool increase also during aging. In fact, in healthy adults, total monocyte levels do not change with age, but after the age of 50 there is a shift from classical CD14++CD16- monocytes to CD14+CD16+ cells. This could represent a physiological trick to preserve the overall functionality of the monocyte pool, but it does not necessarily correspond to enhanced monocyte availability and functionality in aging. Indeed, after the age of 50 the functionality of CD14+CD16+ monocytes seems to decrease. They express lower levels of activation markers and display a potentially reduced capacity to migrate to inflammatory sites and to the spleen. Moreover, the half-life of monocyte-derived tissue macrophages is lower too. Finally, CD14+CD16+ monocytes that are expanded after the age of 50 are generally believed to have the capacity to produce higher levels of pro-inflammatory cytokines. Specifically, advanced-age, frail elderly MONOCYTES demonstrate a higher production of TNF-α, and to a lesser extent IL-8. Pro-inflammatory cytokine production is constitutively higher, suggesting a predisposition to chronic disease development.
Looking at the differences between adults (19-59 years), seniors (61-76 years), and the advanced-aged, frail elderly (81-100 years), the latter showed a significant reduction in CD14++CD16- and CD14+CD16+ monocytes, and an increase in CD14++CD16+ cells. Overall, data in the scientific literature indicate a decrease in CD14++CD16- monocytes and an increase in CD16+ subpopulations starting from 61 years of age. CD14+CD16+ cells seem to increase after the age of 50, but their functionality is compromised; starting from the age of 81, frailty is associated with their reduction, whereas CD14++CD16+ monocytes increase. CD16+ cells, specifically non-classical monocytes, seem to account for the increase in plasma TNF-α levels and inflammatory conditions in aged individuals. Monocyte frequency, phenotype, and functional changes observed in the advanced-age, frail elderly correlate with chronic diseases associated with a chronic inflammatory state. Interestingly, the reversal of this CD16+ cell increase seems to be possible. In obese individuals, for example, both dietary intervention and gastric bypass surgery result in the decrease of these subsets of monocytes.