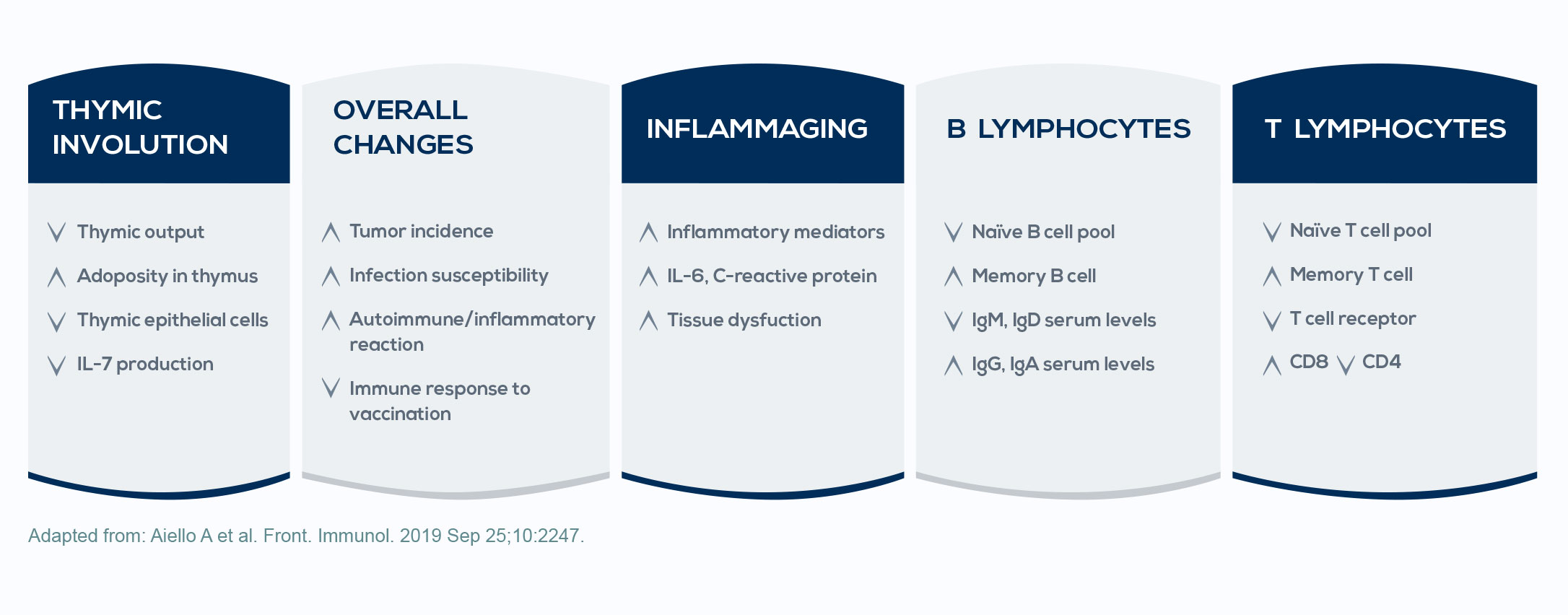
Immune system stimulation and oxidative stress exposure occurring over the course of life promote a state called inflammaging; that is, inflammation associated with aging. This low-grade inflammation status is associated with almost all the health problems typical of aging, including cancer. It is due to chronic immune system stimulation, which induces the increase of both activated immune cells and pro-inflammatory lymphokine production, contributing to the imbalance between inflammatory and anti-inflammatory mechanisms. Moreover, during the course of life, oxidative stress damages cell lipids, proteins, and nucleic acids, triggering protective mechanisms; however, as oxidative insult progresses, such protective mechanisms become less effective. The consequent accumulation of senescent, dysfunctional, and mutated cells leads to a reduction in the immune response and to an increased risk of cancer.
Besides the increased tendency for inflammation, aging also comes with immune defense decay. This phenomenon, known as immunosenescence, is associated with the reduction of the adaptive immune response, the enhancement of the susceptibility to infections, the increase in the production of autoantibodies (that is, antibodies directed against structures of the organism), and increased mortality. This may explain why some diseases involving the immune system (such as malignancies) develop during aging.