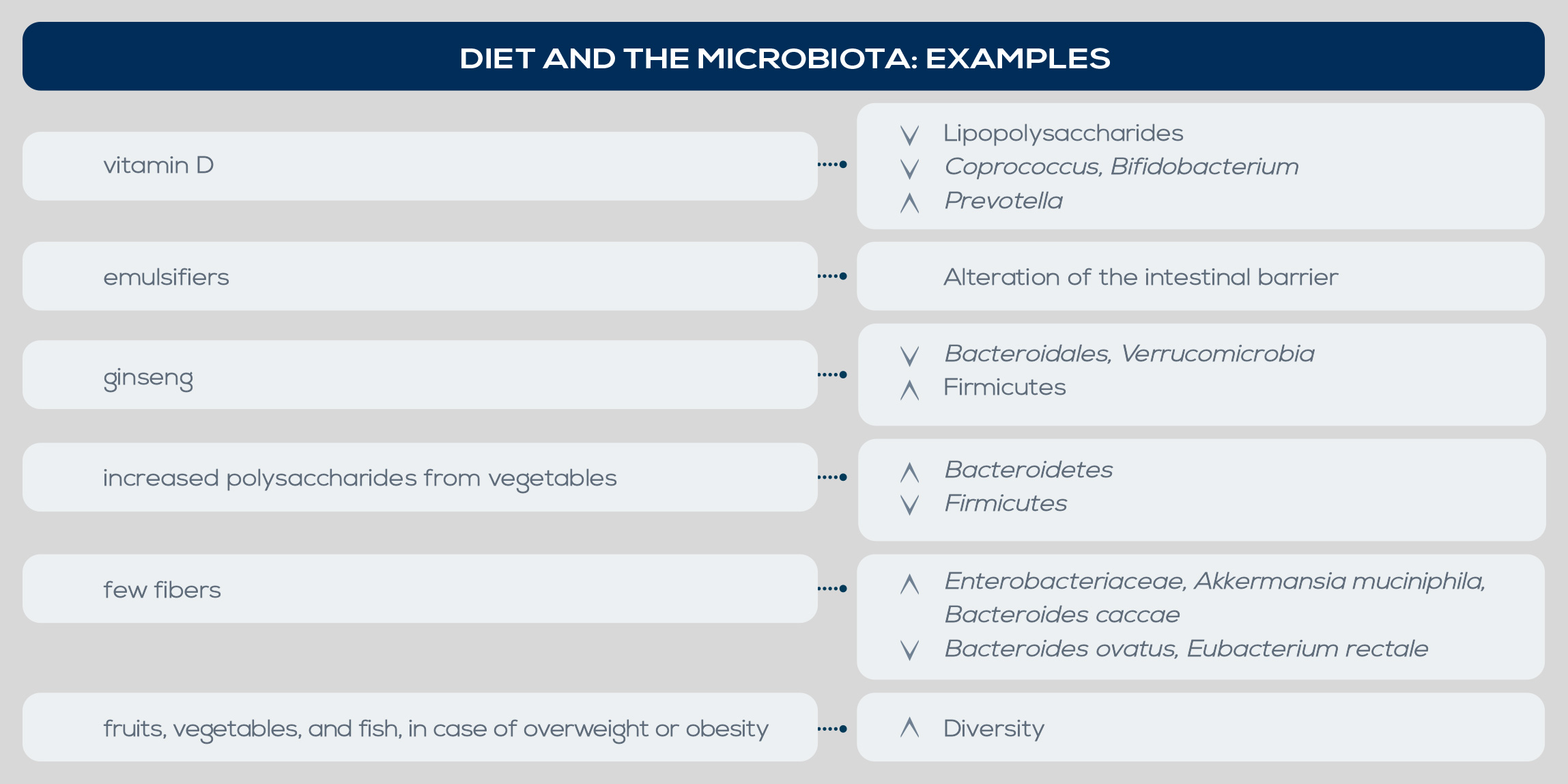
Both obesity and its comorbidities are associated with changes in gut microbiota. In general, obesity is associated with a lesser diversity and richness of gut microbiota, and in obese people the ratio of Firmicutes to Bacteroidetes seems to be higher. Moreover, some studies highlighted a decrease of Methanobrevibacter smithii in obese individuals and a strong association between obesity and Blautia hydrogenotorophica, Coprococcus catus, Eubacterium ventriosum, Ruminococcus bromii, Ruminococcus obeum, and Lactobacillus reuteri, as opposed to a larger proportion of Bacteroides faecichinchillae, Bacteroides thetaiotaomicron, Blautia wexlerae, Clostridium boltae, Flavonifractor plautii, and Bifidobacterium and Lactobacillus species in lean people. Low bacterial richness is also associated with obesity-related conditions, in particular insulin resistance and dyslipidemia.
Insulin resistance has been associated with Lactobacillus increase and Clostridium reduction, and in type II diabetes, the disease can be exacerbated by chronic inflammation driven by complex interactions between the immune system and gut microbiota. SCFA-producing Clostridia strains are reduced whereas E. coli increases, and blood translocation of bacterial products may rise – as suggested by increased serum lipopolysaccharide (LPS) levels. An in-depth study of the link between obesity and gut microbiota has been conducted mainly in animal models, but several data suggest that similar relationships are present in humans too. Among the proposed mechanisms is the ability of gut microbes to regulate energy intake by fermenting dietary fibers. Due to the higher presence of enzymes for complex carbohydrate degradation and fermentation, obesity-associated gut microbiota is characterized by an increased capability to harvest energy from the diet.