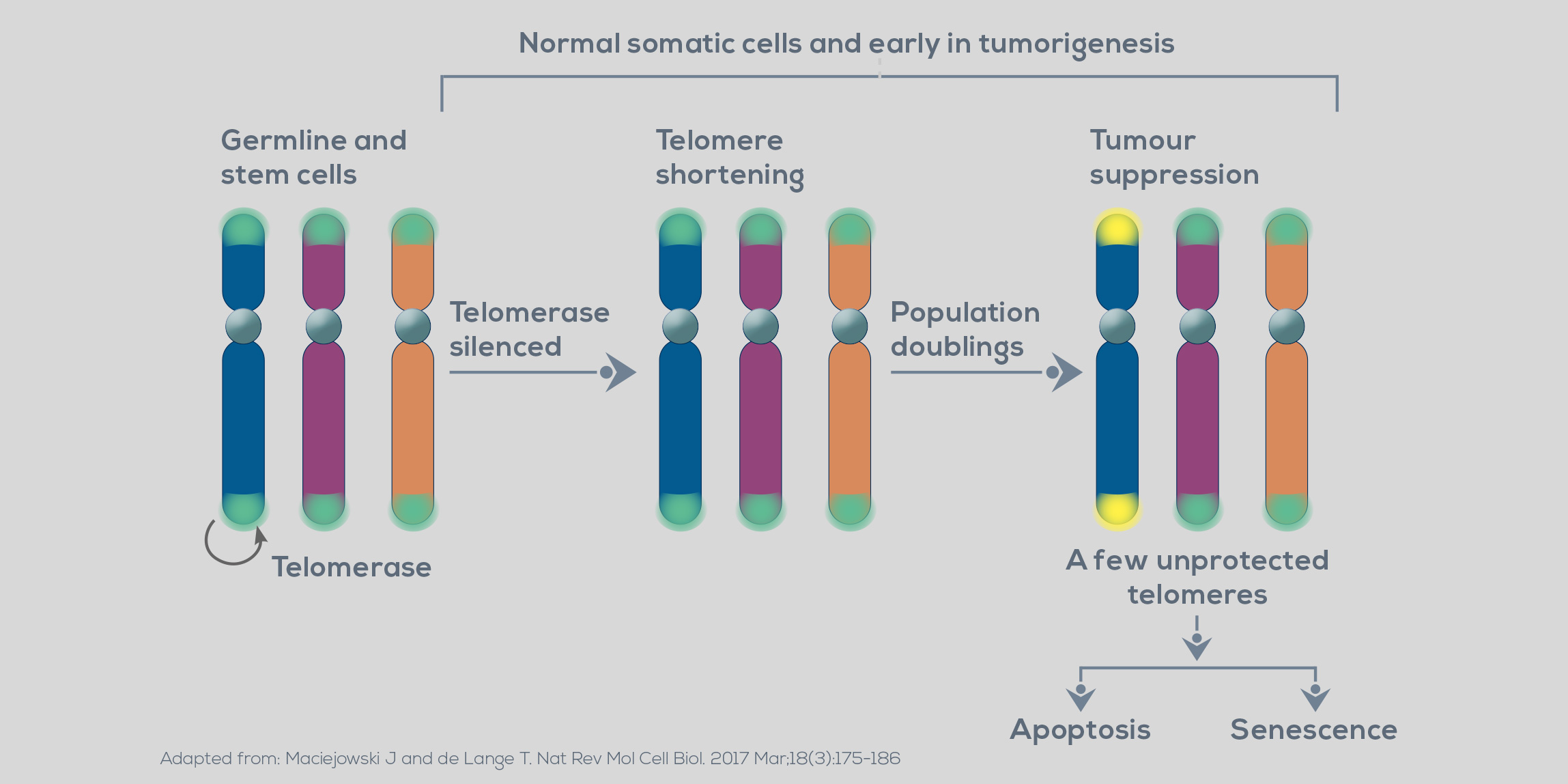
Telomeres represent both genomic sacrifice zones, which prevent the loss of genomic information at the end of chromosomes, and high order structures masking chromosome ends from being detected as a site of damaged DNA by the DNA damage response machinery. Also, telomeres regulate gene expression, transcriptionally silencing genes both close to them and/or at long distances. These structures are characterized by the presence of a terminal single- Human telomere length encompasses a normal range with discrete upper and lower limits. Both longer and shorter telomeres have been linked to cancer. In the first case, the association lies in the presence of mutations promoting telomere elongation. Longer telomeres stranded DNA overhang which folds back invading the preceding repetitive homologous double-stranded region, forming a telomeric loop (T-loop). This conformation protects the otherwise exposed chromosome end from being recognized as a site of DNA cleavage.
Also, the proteins complexed with DNA (TRF1, TRF2, RAP1, TIN2, TPP1, and POT1, collectively named the shelterin complex) binds the repetitive doublestranded sequences and the singlestranded overhang and allows the DNA may favor a delayed cell senescence, increasing the opportunities to develop genomic instability, thus the risk of carcinogenic transformation. On the other hand, shorter telomeres can be a direct source of genomic instability. Unstable, dysfunctional telomeres are recognized as DNA damage repair machinery to distinguish telomeres from sites of DNA breakage. Other higher-order DNA conformations, such as four-stranded structures (the so-called G-quadruplexes), and a long non-coding RNA transcribed from telomere DNA (the telomere repeatcontaining RNA) are thought to be implicated in normal telomere function and maintenance. Human telomere length is heterogenous. Cell viability and chromosome stability depend on the shortest telomere.